Freelance medical writing involves creating various scientific documents, such as research papers, clinical research writing, medical education content, and regulatory papers for various clients in the healthcare industry.
As the worldwide healthcare industry continues to expand and evolve, the demand for skilled medical writers is also on the rise and is only expected to grow in the coming years.
In this article, I provide a detailed overview of the discipline of medical writing and its many specialisms, explain the role of a freelance medical writer, and outline the essential skills you must possess to enter this fascinating field.
I also delve into the regulatory guidelines in medical writing, show you how to create a medical writing portfolio, and discuss the intricacies of navigating the legal and ethical considerations of medical writing.
So, keep reading to learn everything you need to know about becoming a freelance medical writer and building a career in this rapidly growing specialist field.

What Is Medical Writing?
Medical writing is a highly specialized form of technical writing that entails creating documents for the medical, pharmaceutical, and healthcare industries.
This form of writing involves researching and preparing a range of documents, including scientific manuscripts, regulatory documents, clinical study reports, marketing materials, and medical educational content.
What Are the Roles and Responsibilities of a Medical Writer?
A medical writer’s main responsibility is to craft accurate, concise documents that are clear, easy to understand, and based on scientific evidence. The role involves researching, writing, editing, and reviewing medical documents, including clinical study reports, manuscripts, regulatory documents, and other scientific publications.
As a freelance medical writer, you might also be asked to develop scientific content for medical communications, including abstracts, posters, and slide decks.
Precision and accuracy in medical writing are critical since even minor mistakes or omissions can potentially have serious consequences, like misinterpretation or miscommunication of essential clinical information. Therefore, as a freelance medical writer, you must ensure that all documents are well-researched, accurate, and of the highest standard.
What Are the Specialization Areas in Medical Writing?
As mentioned above, medical writing is a highly specialized field with many different avenues to go down for freelancers with relevant experience and skills.
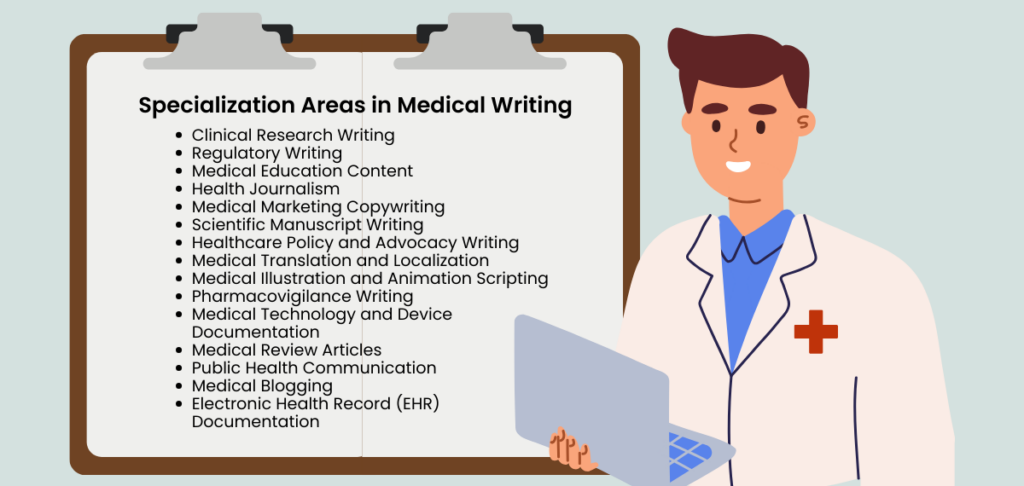
Clinical Research Writing
Clinical research writing involves creating various documents related to clinical research studies. That can include study protocols, case report forms, clinical study reports, informed consent forms, and manuscripts for publication. Your goal as a freelancer is to ensure that the data is collected accurately and that the results are reported clearly and transparently.
Regulatory Writing
Regulatory writing is a specialized field of medical writing where you create documents for regulatory agencies such as the Food and Drug Administration (FDA) or the European Medicines Agency (EMA). These documents are typically required to approve drugs, medical devices, and other healthcare products.
Medical Education Content
Medical education content typically refers to information relating to anatomy, physiology, pathology, pharmacology, and clinical skills. As a freelance medical writer, you might be required to present that information in various formats, including textbooks, lectures, case studies, simulations, and interactive modules.
Medical education content can cover topics related to diagnosing, treating, and managing various medical conditions such as diabetes, hypertension, cancer, and infectious diseases.
Health Journalism
Health journalism involves researching, writing, and reporting on various health-related topics for different types of media outlets, including newspapers, magazines, websites, and TV/radio broadcasts.
As a health journalism writer, your goal is to educate your readers about health issues by providing accurate, evidence-based information that empowers them to make informed decisions about their health.
To work in medical writing, you need strong research and writing skills and excellent communication skills to effectively convey complex medical information in layman’s terms to a general audience.
Medical Marketing Copywriting
As a medical marketing copywriter, you create promotional content for various healthcare products and services. Your goal is to persuade the target audience, which could be healthcare professionals, patients, or consumers, to choose the product or service being promoted.
Scientific Manuscript Writing
Scientific manuscript writing involves preparing a well-structured manuscript that presents research findings and conclusions in a clear and concise manner.
The main aim of scientific manuscript writing is to report research findings to the scientific community and beyond. A well-written scientific manuscript usually includes an abstract, introduction, methods, results, discussion, and conclusion sections.
You’ll find examples of scientific manuscripts in many scientific journals, including Nature, Science, and PLOS One.
Healthcare Policy and Advocacy Writing
Healthcare policy and advocacy writing involves creating documents that influence healthcare policies, laws, and regulations. These documents aim to bring about positive changes in the healthcare system and ensure that people receive the best possible care.
Medical Translation and Localization
This kind of writing refers to translating and adapting medical documents, information, and content from one language to another while accurately maintaining the intended meaning and context. That’s critical in the healthcare industry because it ensures that patients and medical professionals can communicate effectively regardless of language barriers.
Medical Illustration and Animation Scripting
This form of medical writing involves creating illustrations and animations to help medical professionals and patients better understand complex medical procedures, anatomy, and diseases in a way that’s easy to understand.
For example, medical illustration and animation scripting jobs can include creating 3D animations of surgical procedures or crafting illustrations or infographics to explain the structure and function of the human body.
Pharmacovigilance Writing
Pharmacovigilance writing is critical to drug safety and regulatory compliance. This form of writing involves creating various documents, including clinical studies, adverse event reports, and risk management plans, to ensure that drugs are safe and effective for patients.
To undertake this kind of medical writing, you must thoroughly understand medical terminology and clinical research.
Medical Technology and Device Documentation
This field of medical writing involves working on various types of documents, such as user manuals, instructions for use, labeling, and technical specifications for medical devices.
Writers in this niche need a thorough understanding of the product, its intended use, and the regulatory requirements in the country or region where it will be marketed. They work closely with product development teams, regulatory affairs professionals, and quality control experts to produce accurate, comprehensive documentation that meets all regulatory requirements.
Medical Review Articles
Medical review articles provide an overview of current knowledge on a specific medical topic. They are often published in peer-reviewed medical journals and are written by experts in the field.
If you’re a freelancer working in this field, you’ll need a strong background in the subject matter and a thorough understanding of the scientific research and literature on the topic.
Public Health Communication
Public health communications are designed to educate the public about health-related issues, like disease prevention, health promotion, and healthcare services.
Creatives working in this area must strongly understand the target audience and the health issue being addressed. Public health communication writers typically work on content for social media, public service announcements, and community outreach programs.
Medical Blogging
Freelancers focusing on medical blogging work on creating online content for various healthcare-related topics, including disease prevention, treatment options, healthcare trends, and personal experiences with illness or healthcare.
To work in this field, you need a good understanding of the subject matter and excellent writing and research skills. The content you create must be engaging, informative, and authoritative to resonate with your readers and keep them coming back to the site for more.
Electronic Health Record (EHR) Documentation
Working on Electronic Health Record (EHR) documentation involves creating and updating patient medical records in electronic format.
Freelance writers in this field must have a strong grasp of medical terminology, data privacy laws, and regulations governing EHRs. They work closely with healthcare providers, IT professionals, and compliance teams to produce concise documents that maintain patient confidentiality.
What Essential Skills Do Freelance Medical Writers Need?
If you embark on a new career as a freelance medical writer, here are some essential skills you’ll need to succeed.
Medical Terminal Mastery
Medical writers must have a good working knowledge of the medical terminology used in their chosen specialist area. For example, when I worked as a medical writer in an Ear, Nose, and Throat clinic, I quickly became familiar with all the terms used by the consultants I worked with.
That’s essential since some of the words used are very similar, but a misspelling could completely change the context and meaning of a document.
Research Proficiency
By its very nature, freelance writing demands proficiency in researching reliable sources for useful information and citing them accordingly in your work. That’s especially important for medical writers, as misinformation or incorrect data could render a document useless or misleading.
Critical Appraisal Skills
Critical appraisal skills are essential for freelance medical writers because they allow them to gather, assess, and interpret information from scientific studies and published literature.
Adaptability
As a medical writer working on medical blogs and across multiple fields, you’ll need to show your adaptability by demonstrating your ability to research and write about different medical disciplines.
Effective Communication
For freelance medical writers, effective communication with medical professionals and their patients is essential. You must also be able to discuss your research findings with experts in the field to establish their validity and reliability.
Attention to Detail
As with any form of content creation, you must have a keen eye for detail and be able to rule out typographical, formatting, and grammar issues in your work. Attention to detail is also essential for effective research, especially in highly specialized medical fields.
Time Management
As a freelancer, you must be able to manage your time effectively to hit your clients’ deadlines. That’s especially important when working on delivering important healthcare messages across social media and in medical publications where lead times are critical.
Medical Writing Software Proficiency
All medical writers must be familiar with and proficient in using software designed specifically for use in their chosen medical discipline. Of course, understanding how to create and use spreadsheets and accounting software is also essential for your own record-keeping.
Regulatory Knowledge
The medical and pharma industries are heavily regulated, and you must understand the rules and regulations that apply to the content you’ll produce for your chosen niche.
Collaboration and Teamwork
Throughout your career as a freelance medical writer, you’ll work with healthcare professionals and other workers within hospitals and clinics. To do that effectively, you must be able to work as part of a team and collaborate with others to produce the best content you can.
Ethical Considerations
When working on some medical documents, you’ll be privy to patients’ confidential information. In addition, if you work on research papers and new drug documentation, you might see some data that could be highly confidential.
For that reason, you must have strong ethics that prevent you from commenting on or divulging that data to anyone outside your professional medical team.
Adherence to Style Guides
To produce correctly formatted and presented medical writing, you’ll need to have a thorough knowledge of the appropriate style guide for your specific niche.
Storytelling Skills
By using storytelling techniques, you can simplify medical jargon and put across confusing information in a way that is easy for patients to understand. You can also use anecdotes, case studies, and patient testimonials to make your work more relatable and memorable, which is especially important when communicating health information to lay audiences.
Adaptation to New Technologies
Technology is always moving forward, and that’s certainly the case in healthcare and medicine. As a freelance medical writer, it’s essential to keep up to speed with new technological developments, such as new writing software, and adapt to them.
Client Relationship Management
A key skill for all freelance writers is the ability to manage client relationships effectively. That can mean knowing how to negotiate rates, pitch for new projects, and establish yourself as the client’s go-to writer when they need someone to work on an urgent project.
How to Navigate Regulatory Guidelines in Medical Writing
As previously mentioned, the medical industry is heavily regulated, and you’ll need to know how to navigate the regulatory guidelines related to medical writing.

Key Regulatory Bodies
A number of regulatory bodies play a crucial role in safeguarding public health. They do that by ensuring that new drugs and medical devices are thoroughly evaluated before reaching the market and by monitoring their safety and effectiveness thereafter.
As a freelance medical writer, you should understand the guidelines set out by these regulatory bodies.
FDA (U.S. Food and Drug Administration)
The FDA is one of the most prominent regulatory agencies globally and is responsible for regulating drugs, medical devices, food, cosmetics, and tobacco products in the United States.
The Administration is responsible for reviewing manufacturers’ applications for approval of new drugs and medical devices. It also conducts rigorous clinical trials of these products to assess their safety and suitability.
The FDA evaluates data submitted by pharma and medical device companies, inspects manufacturing facilities, and decides whether to approve or reject applications for marketing authorization.
In addition, the FDA monitors the post-market safety of drugs and devices, issuing warnings and recalls as necessary to protect public health.
EMA (European Medicines Agency)
The EMA is responsible for evaluating and supervising medicines for human and veterinary use in the European Union (EU) and European Economic Area (EEA).
Like the FDA, they evaluate preclinical and clinical data to see that products are safe, effective, and of good quality. The EMA also provides scientific advice to manufacturers throughout the drug development process and monitors the safety of medicines post-approval.
Other Regional Regulatory Agencies
Other countries and regions have their own regulatory agencies responsible for overseeing the approval and regulation of pharmaceuticals and medical devices. These agencies essentially do the same job as the FDA and EMA.
For example, Japan has the PMDA (Pharmaceuticals and Medical Devices Agency), and Australia has the Therapeutic Goods Administration (TGA). In India, the CDSCO (Central Drugs Standard Control Organization) functions under the Directorate General of Health Services, Ministry of Health & Family Welfare, Government of India.
Regulatory Documents
There are several regulatory documents you could work on as a freelance medical writer:
Clinical Study Protocols
Clinical study protocols outline a clinical trial’s objectives, design, methodology, and statistical analysis. They include participant inclusion and exclusion criteria, primary and secondary endpoints, a statistical analysis plan, and ethical and legal considerations.
Clinical Study Reports
These comprehensive documents provide a detailed summary of a clinical trial’s design, methodology, and results.
CSRs play a critical role in the approval process for new drugs, medical devices, and other healthcare products and provide regulatory agencies, such as the FDA or EMA, with crucial information about the product being tested.
Investigator Brochures (IBs)
An Investigator Brochure (IB) provides clinical investigators with information about a drug or medical device under investigation. This critical document ensures clinical trials are conducted safely and effectively and is also essential to regulatory authorities responsible for approving the drug or device.
Common Technical Document (CTD) Format
The CTD is a document in a standardized format for submitting information to regulatory agencies in the pharmaceutical and biotechnology industries.
This document comprises five modules that organize and present relevant data related to the drug or biologic being submitted for regulatory approval. The structured format makes it easier for regulatory authorities to review and approve the submission.
Adherence to Guidelines
- Following the ICH guidelines established by the International Council for Harmonisation of Technical Requirements for Pharmaceuticals for Human Use is essential. These guidelines ensure that pharmaceutical products are safe, effective, and of high quality.
- Good Clinical Practice (GCP) is a set of principles designed to ensure scientifically sound, ethical clinical research. That provides a framework for clinical trials involving human subjects. The principles of GCP are based on the belief that trial subjects’ rights, safety, and well-being should always be the top priority. That includes:
- obtaining informed consent from trial participants
- ensuring their safety
- conducting the study by approved protocols
- maintaining confidentiality of participant data
- ensuring the accuracy and integrity of all data collected during the study.
Precise and Accurate Data Presentation
As a freelance medical writer, you must understand the importance of precise data presentation and visualization techniques in navigating regulatory guidelines.
That’s especially true regarding statistical analysis and interpretation within regulatory submissions. To ensure data integrity, you must avoid bias or misleading interpretations in reporting. So, paying close attention to proper data visualization and interpretation techniques is essential to ensure that the facts can be easily understood and represented accurately.
How to Build a Medical Writing Portfolio?
You’ll need an impressive medical writing portfolio to pitch to potential clients. Here’s how to build one.
Types of Samples to Include
When compiling your portfolio, you must include examples of your past work and experience to demonstrate your competence in working on particular medical documents. The more information you can provide to potential clients, the more likely you’ll pique their interest.
Be sure to include the following samples in your portfolio:
- Regulatory documents, such as protocol sections, CSR components, and mock IB sections
- Marketing materials, including website copy, brochures, and patient education materials
- Material from scientific publications, like review articles, case studies, and study summaries
- Blog posts and articles demonstrating your expertise in specific areas
Where to Find Projects
One of the biggest challenges for freelance medical writers is finding suitable projects and clients that will provide them with ongoing work. Essentially, the more specialist your niche, the more difficult it will be to secure much work, especially in the early days of your career.
Here are a few places that can be a happy hunting ground for freelance medical writers looking for work:
- Volunteer opportunities with non-profits or health organizations
- “Spec” work or writing mock pieces for fictional products of companies
- Contently and other platforms that allow you to showcase your work, even if unpaid
- Small projects from freelance job boards
Formatting and Presentation
The saying “first impressions count” is true when building a medical writing portfolio. Medical professionals tend to be meticulous in their working practices, so a chaotic, confusing portfolio will not impress potential clients in this demanding field.
- Organize and label all your sample materials clearly, and include an index if your portfolio is extensive.
- Keep your design professional. Remember, even a simple, clean template can make a big difference in the impression you create.
- If you decide to include confidential information about former clients, their patients, or clinical study participants, be sure to redact it. Alternatively, anonymized samples can be created instead of real-life data.
Beyond the Portfolio
You can reinforce the impact of your portfolio by providing more relevant information to catch the attention of potential clients. Don’t be afraid to blow your own trumpet; this is your final chance to impress!
- Highlight relevant skills on your resume/CV, such as research and data analysis.
- Consider including a brief cover letter to explain any gaps in your resume or showcase your passion for your chosen specialist medical niche.
How Can You Network in the Medical Writing Community?
An excellent, effective way of raising your profile as a freelance medical writer is to get busy networking with like-minded individuals.
I strongly recommend joining some relevant industry organizations where you can benefit from access to conferences, workshops, online communities, and job boards.
The American Medical Writers Association (AMWA) and the European Medical Writers Association (EMWA) are good ones to consider. However, many other regional or niche-specific organizations might suit your specialism.
What Are the Legal and Ethical Considerations in Medical Writing?
As you might expect, freelance medical writers must consider some legal and ethical considerations when working closely with confidential personal information and data relating to clinical drug trials.
Accuracy and Transparency
Accuracy and transparency are crucial considerations in medical writing, both from legal and ethical perspectives. As a medical writer, you must ensure that your work is accurate, supported by evidence, and free from conflicts of interest.
Patient Privacy and Confidentiality
When working on certain projects, you might be privy to confidential information about a patient’s health and personal information. That information must always be treated with the utmost care and should never be shared.
Intellectual Property
Intellectual property is an important consideration in medical writing, and as such, you must respect the legal rights of the original creators of a work, including patents, trademarks, and copyrights.
Marketing and Advertising Regulations
When writing marketing or advertising materials for medical products, you must ensure that your work complies with the relevant regulations. In the US, the FDA regulates the marketing of medical products, while in Europe, it’s the EMA.
Ghostwriting and Authorship
When you’re working as a freelancer, some of your work will be ghostwritten and some authored. The two are different things.
A ghostwriter writes on behalf of someone else without receiving any credit for the work, while an author is credited for writing the content. In most cases, ghostwriters receive payment for their work. An author will often be paid and receive royalties for their work, depending on the subject matter, for example, when writing a book.
When creating a portfolio, always try to include as much credited, authored work as possible.
Conclusion
This guide highlights the increasing demand for freelance medical writers across various niches in the healthcare industry.
As a medical writer, you must produce accurate, clear writing that adheres to the relevant regulatory guidelines for your region or country. Depending on your experience, you might want to specialize in a particular area, such as clinical research, regulatory writing, medical education content, health journalism, and medical marketing copywriting.
To succeed in this challenging field, you’ll need various essential skills, from mastery of medical terminology to regulatory knowledge and ethical considerations. You’ll also need to be able to navigate regulatory guidelines and address legal and ethical considerations in medical writing.
Use credited writing pieces to build a professionally presented medical writing portfolio that sells your skills to potential clients. Be sure to get busy networking in the medical writing community to find work.
